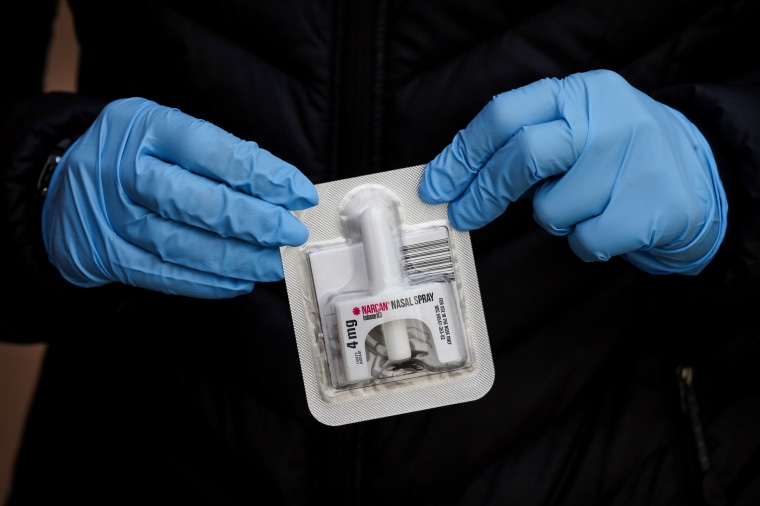
The Food and Drug Administration is pushing for drugmakers to apply for over-the-counter approval for naloxone, the opioid overdose antidote.
In a notice published online Tuesday, the agency said that it “strongly encourages” naloxone makers to contact the FDA “as early as possible” to initiate a discussion about a potential switch from prescription to over the counter.
It's a move, experts say, that would remove barriers to accessing the life-saving medication, which can reverse the effects of an opioid overdose when given in time. The FDA said that two forms of the drug — a nasal spray and an autoinjector — could potentially be safe and effective for over-the-counter use.
Naloxone is only offered as a prescription, though many states have found work-arounds to make the drug easier to get. Through so-called standing orders, for example, people can request naloxone from a pharmacist.
By making the medication available over the counter, it removes stigma, said Dr. Scott Hadland, an addiction specialist at Mass General for Children in Boston. People would be able to purchase it online or in store via self-checkout.
There’s been a big push from advocacy groups over the past few years to make the drug more easily available, said Dr. Michael Barnett, an assistant professor of health policy and management at the Harvard T.H. Chan School of Public Health.
More than 107,600 Americans died from drug overdoses last year, according to the Centers for Disease Control and Prevention, the highest annual death toll on record. The majority involved fentanyl, a powerful synthetic opioid. Nearly 20,000 deaths from overdose, between 1999 and 2020, have been prevented by the self-administering of naloxone, according to the FDA.
The FDA’s notice provides a pathway to approval for the products and calls for more information from drugmakers on how the medication would be sold. Over-the-counter naloxone would still need to be submitted to the FDA for review and approval, Barnett said, but could start rolling out onto drugstore shelves as early as next year.
The agency specifically mentioned two products that could be potential candidates for over-the-counter use: up to 4 milligrams of Narcan, a nasal spray from Emergent BioSolutions, and up to 2 milligrams of Evizo, a single-use autoinjection from Kaleo. These products are easy to use, the agency wrote, because they don't require additional supplies or medical training.
Naloxone products that come in higher doses and other forms of the drug for which more safety data is still needed, such as those that come in glass vials and ampules, would still need to be prescribed by a physician, the FDA said.
Despite efforts from many states to make naloxone more widely available, barriers remain.
Some patients might be shy about asking a pharmacist for the medication, because of the stigma surrounding addiction. More than 21 million people in the U.S. suffer from an addiction, according to the Association of American Medical Colleges, and just 11% of them receive the treatment they need.
Additionally, some pharmacists may not be aware that there is a standing order in their state and refuse to prescribe the drug altogether.
Hadland said he’s also seen patients get denied life insurance coverage after the medication appeared on their health insurance bill and the life insurance provider believed they were at risk for an overdose.
Making the drug available over the counter also means people wouldn't have to rely on the protection of Good Samaritan legislation when administering the drug, said Dr. Anna Lembke, the medical director of addiction medicine at Stanford. These laws offer limited legal protection to people who help someone who is injured or ill.
“If it were to be made over the counter, all of that would be moot and the average person could buy it without a prescription and without needing legal protection to administer,” she said.
The FDA noted that over-the-counter naloxone will not divert supplies away from community-based naloxone distribution programs and hospitals.
Follow NBC HEALTH on Twitter & Facebook.